‘Future of cancer treatment’ has just been approved

Reproduced from The Australian
7 June 2023
Natasha Robinson – Health Editor
A new treatment, dubbed “the future of cancer therapy”, has been approved to treat a type of blood cancer in a step doctors are hailing as the beginning of a “total paradigm shift” in cancer care for the sickest patients who have failed to respond to conventional treatments.
The therapy, CARVYKTI, has been approved by the Therapeutic Goods Administration to treat people with multiple myeloma who have not been able to combat their cancer with three previous lines of other treatments.
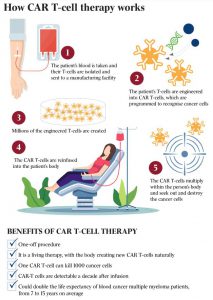
The treatment is the first approval for a common cancer in Australia of CAR T-cell therapy, a type of personalised immunotherapy that engineers a patient’s own T-cells to kill cancer cells. The therapy is a “one-off” treatment that provides a functional cure for cancer.
The Australian scientists pioneering the treatment say the therapy could result in 80 per cent of cancers being curable within two to three decades, with an estimated 4.5 million cases of the disease expected to be diagnosed in the next quarter-century.
Patients who receive CAR T-cell therapy have an amount of blood taken from their body and sent to a special laboratory, where their own T-cells are “re-engineered” to carry special structures called chimeric antigen receptors (CARs) on their surface. When these CAR T-cells are reinjected into the patient, they multiply rapidly, and the engineered receptors help the patient’s own T-cells identify and attack cancer cells throughout the body.
“This therapy represents a total paradigm shift,” said Miles Prince, director of the Centre for Blood Cell Therapies at the Peter MacCallum Cancer Centre and director of Molecular Oncology at the Epworth Hospital in Melbourne. “It’s a one-off living drug that can cure patients that have cancers that are otherwise incurable.
“This approval is really important news and it’s the first major step towards regulation of this product. It sets the scene for us to be able to establish this therapy in Australia, and we can start … preparing for the treatment of patients with myeloma.”
The TGA confirmed to The Australian it had granted approval to CARVYKTI, which also goes by the generic name cilta-cel, and that the therapy will be published imminently on the Australian Register of Therapeutic Goods. The therapy has been granted approval for use in patients who have received at least three prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 antibody.
However, the approval does not mean ready access to the therapy for patients as the Medical Services Advisory Committee has previously refused to approve it to be publicly funded.
Pharmaceutical company Janssen confirmed it would lodge an urgent reimbursement application to the MSAC for consideration in its November meeting.
Multiple myeloma is a type of blood cancer that develops from plasma cells in the bone marrow. Around 1100 people who lose their lives to multiple myeloma are diagnosed in Australia every year, and the estimated five-year survival rate is 55 per cent. CAR T-cell therapy is estimated to be indicated for around 880 multiple myeloma patients a year.
Phil Citera received the therapy as part of a clinical trial after being diagnosed with multiple myeloma at the age of 44 and having failed previous treatments. “I had lesions of cancer all over my body from head to toe, they were just everywhere,” he says. “I was struggling to walk. I remember saying to my wife ‘if this doesn’t work I don’t think I’ll be around’.
“But the treatment was a game-changer. The amount of lives this will save if they give it the funding, it definitely stands up.”
There are more than 100 clinical trials under way testing the efficacy of CAR T-cell therapies “for a vast array of indications” including many cancers, auto-immune disease and even infectious diseases. Three other CAR T-cell therapies have already been approved in Australia for very rare types of lymphoma, but the approval of CARVYKTI marks the first time the therapy has been approved for a common cancer.
Haematologist Simon Harrison, who recently helped author a report calling on the federal government to embrace and fund CAR T-cell therapy, said the treatment was expensive but had very high rates of success.
“The results are really transformative,” Professor Harrison said. “If you look at alternative standard of care therapies currently approved in Australia, it’s like chalk and cheese.”
The Evohealth report said there was “compelling evidence” from clinical trials that between 72 and 97 per cent of multiple myeloma patients respond to CAR T-cell therapy and that up to 77 per cent of patients remained free of disease progression at 12 months.
“There is an urgent need for Australian governments to invest in funded access to CAR T-cell therapy for patients with multiple myeloma,” the report said.
The efficacy of cilta-cel as an earlier treatment line in patients with multiple myeloma was confirmed in research published this week in the New England Journal of Medicine, where a peer-reviewed study involving Australian scientists found the therapy could halt the progression of multiple myeloma by three-quarters.
The study, presented at the American Society of Clinical Oncology in Chicago, found CARVYKTI cut the risk of disease progressing by 74 per cent in people whose myeloma was no longer responsive to lenalidomide, a chemotherapy medicine.