Forty per cent of patients with the most common form of aggressive non-Hodgkin lymphoma (diffuse large B-cell lymphoma) either relapse or are refractory to first line treatment with rituximab and chemotherapy. Stem-cell transplant was the only available treatment option thought to reliably induce cures until the approval of chimeric antigen receptor (CAR) T-cell therapy. However, … Continued
We are very proud to announce that the Snowdome Foundation has earned a place in the High-Growth Companies Asia-Pacific 2021 ranking. The ranking assesses millions of companies from 13 countries and Snowdome made it into the top 500 fastest-growing companies in the region. Only 71 companies from Australia made into the top 500 and we … Continued
Multiple myeloma has been a notoriously difficult blood cancer to manage. Relapses are common despite the advances that have been made in treatments over the years. Idecabtagene vicleucel (Ide-cel) a new chimeric antigen receptor (CAR) T-cell therapy, has just been approved by the FDA for relapsed refractory multiple myeloma. This is the first CAR T-cell … Continued
For anyone living with a blood cancer, chimeric antigen receptor T-cell therapy (CAR-T) is a lifeline. Leukaemia and lymphoma patients who have exhausted all other treatment options are given another chance at life. Their own immune system T-cells are genetically engineered to recognise cancer cells and destroy them. It is expected that the demand for … Continued
Myeloma is currently known as a terminal blood cancer. There is no cure, however there are many new treatments on the horizon which show great promise. Clinical trials are the gateway to accessing these new treatments, but not all myeloma patients are created equal when it comes to clinical trial eligibility. Geoff Nyssen wants to … Continued
We are incredibly proud to share with you our 2020 Annual Review featuring financial highlights from 2019-2020. It was a year full of challenges, one that sharpened our focus on what is truly important and dear to us. We believe in our mission to accelerate next-generation treatments for Australian blood cancer patients to help them … Continued
For many patients access to a clinical trial can literally save their life. In speaking to The West Australian newspaper, Prof Chan Cheah told reporter Katie Hampson, when he trained in Melbourne and the U.S he had a range of trials he could put a patient on when they had exhausted all other options. These options … Continued
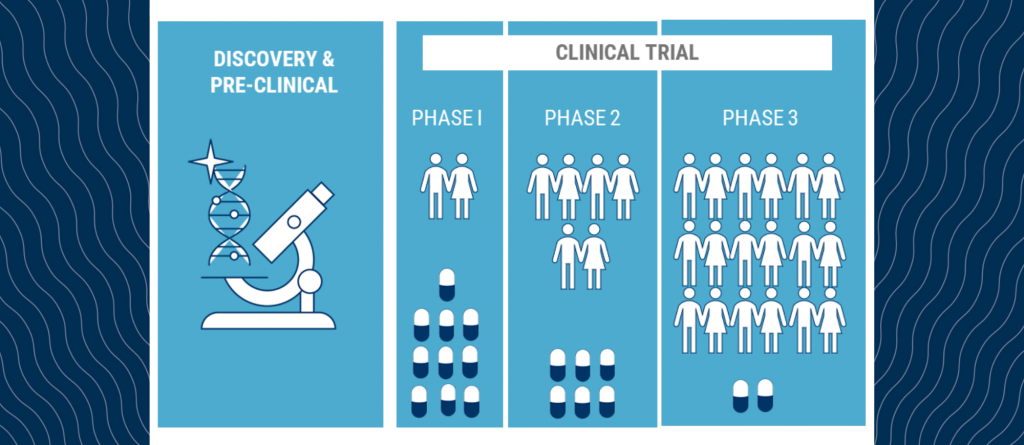
Clinical trials are often the gateway to new treatments for patients with blood cancer. When new therapies are being tested they go through various stages. If you have ever wondered about the various clinical trial stages and what they mean, below is a synopsis to provide you with some insight. Phase I trials are the … Continued
In the last six weeks the U.S. Food and Drug Administration (FDA) has approved five additional treatments for blood cancer. The rate at which new treatments are being approved in the U.S. is incredibly encouraging. It demonstrates the progress researchers are making in finding new and novel ways to treat cancer. It is also frustrating … Continued